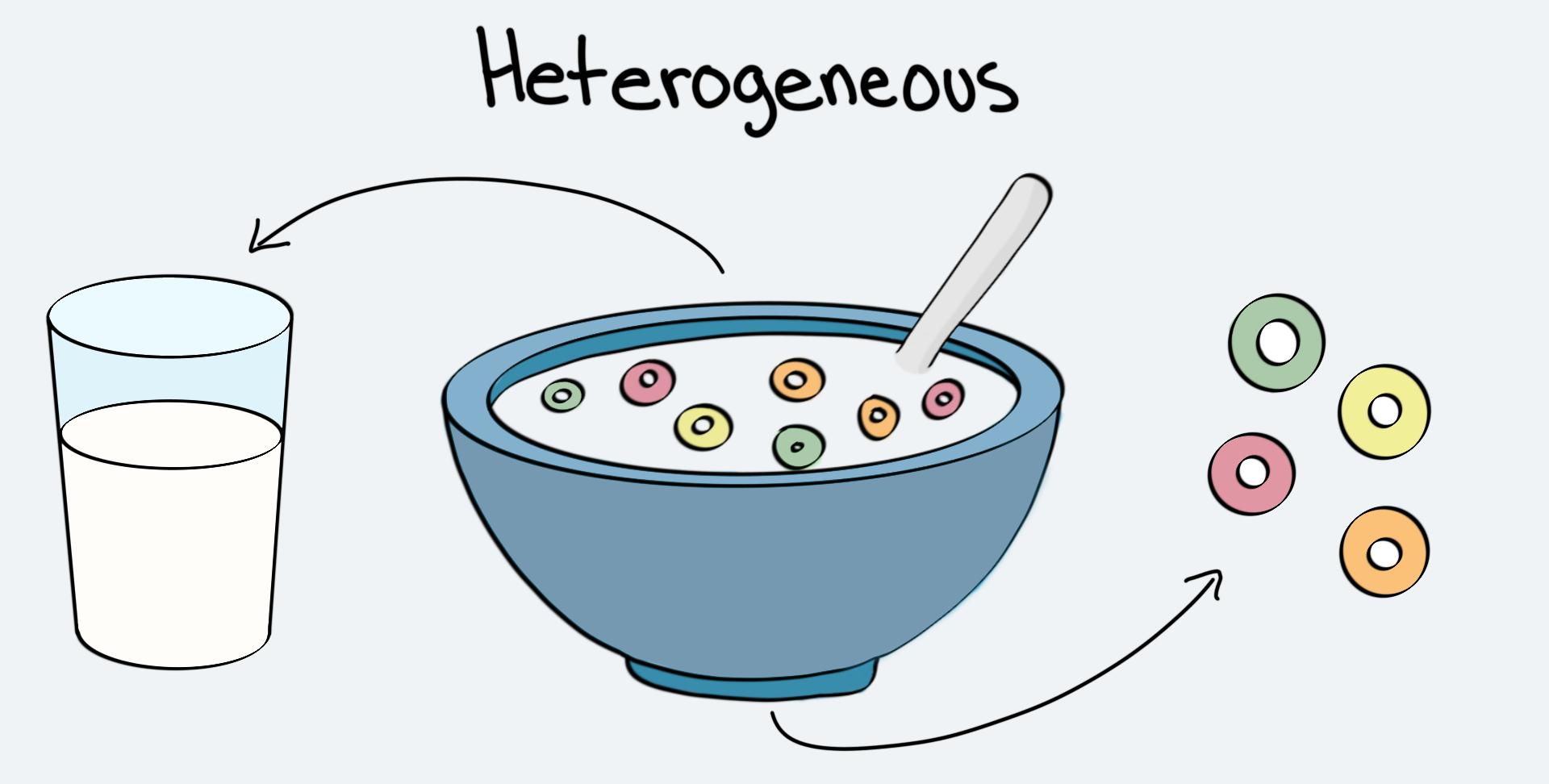
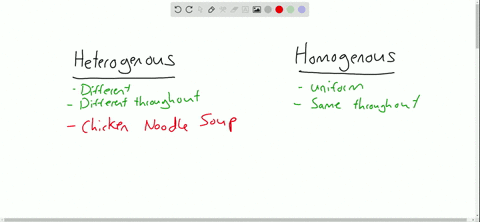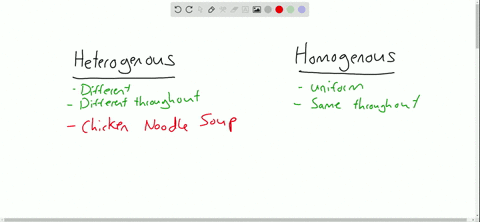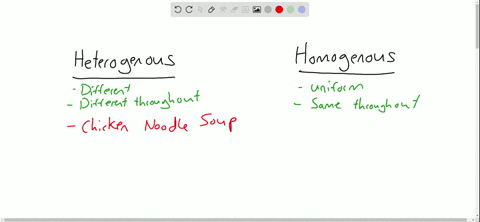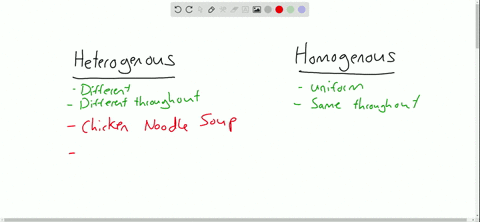
Heterogeneous vs Homogeneous Identify which mixtures are hetero and which are homo Show more Show fewer 2 Num Tries Sensitive Upper/Lower Case Accents Click here to Completely opposite of homogeneous mixtures, heterogeneous mixtures are two or more substances that are distinct from one another For example, the physical eye can pick up the substances that make up this type of mixture because they are large enough to be seen Like homogeneous mixtures, examples of heterogeneous mixtures can include solids Definition A homogenous mixture is the type of mixture in which the composition of the solute is uniform throughout the mixture The heterogeneous mixture is the type of mixture in which the composition of the solute is not uniform throughout the mixture Particle size

Warning This Slide Show Will Make You Hungry
Heterogeneous mixture vs homogeneous mixture worksheet
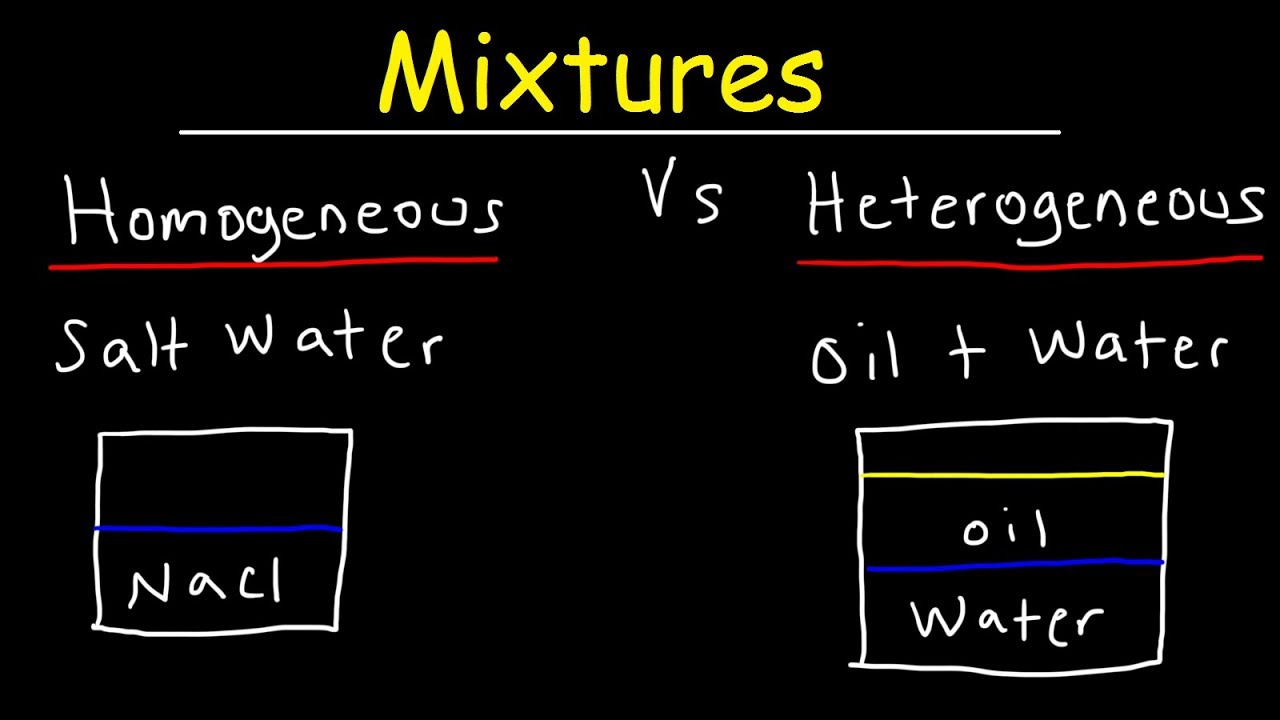
Heterogeneous mixture vs homogeneous mixture worksheet- Main Difference – Homogeneous vs Heterogeneous Mixtures A mixture is a combination of different substances which retain their own characteristics and can be separated by physical means These dissimilar particles do not undergo any chemical transformation while being a part of the mixture A mixture consists of sugar in water is a homogeneous mixture because we can't see particles of sugar in the water, as they are dissolved thoroughly A mixture consisting of oil in water is an example of the heterogeneous mixture as the oil cannot be mixed in the water and we can easily see them I hope you like my post about "Difference




Heterogeneous And Homogeneous Mixtures With Examples Study Guide Brighthub Education
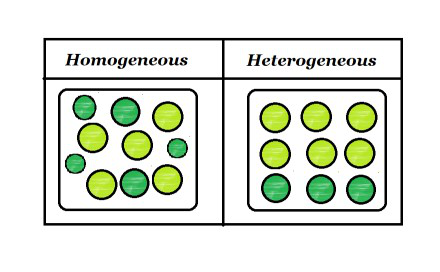
Heterogeneous Mixtures Heterogeneous mixtures mixtures that are NOT evenly mixed! A mixture is formed by combining two or more materials A homogeneous mixture appears uniform, regardless of where you sample it A heterogeneous mixture contains particles of different shapes or sizes and the composition of one sample may differ from that of another sample A solution is mainly of two types that are homogeneous mixtures and heterogeneous mixtures A solution is a homogeneous mixture of solute and a solvent A solute is a substance that gets dissolved in a solvent whereas the solvent is the substance that dissolves a solute in itself
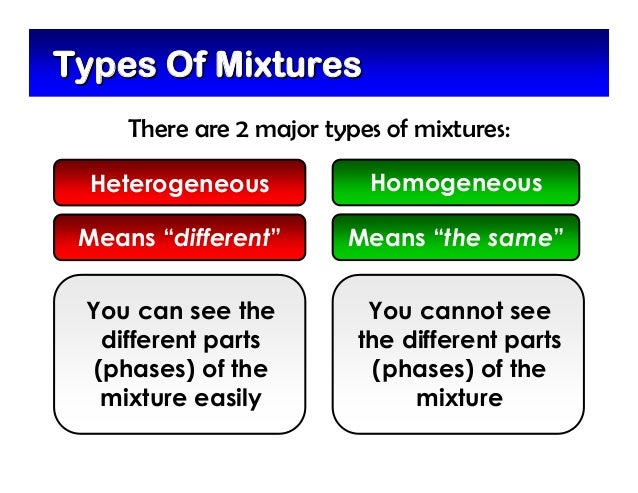
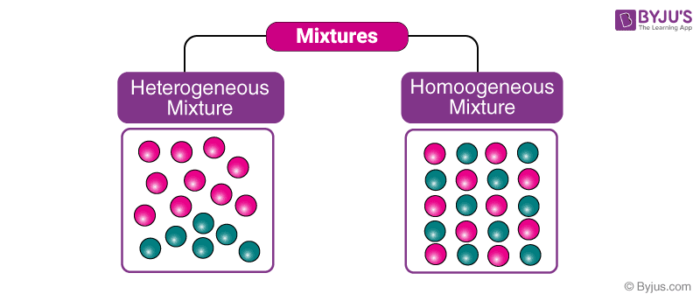
Homogenous vs Heterogeneous Matter created by Simmy8 on 21 Jun 12, enabled by jimmy Sciences Medium level (75% of success) 15 questions 52 037 players Classify the following substances and mixtures as either homogeneous or heterogeneous Homogeneous mixture Heterogeneous mixture It has a uniform composition It has a nonuniform composition It has only one phase There are two or more phases It can't be separated out physically It can be separated out physically 'homo' means the same 'hetero' means different Example a mixture of alcohol and water Heterogeneous products are products with attributes that are significantly different from each other, which makes it difficult to substitute one product for another An example of a heterogeneous product is a computer Commodities are generally a good example of homogeneous products See full answer
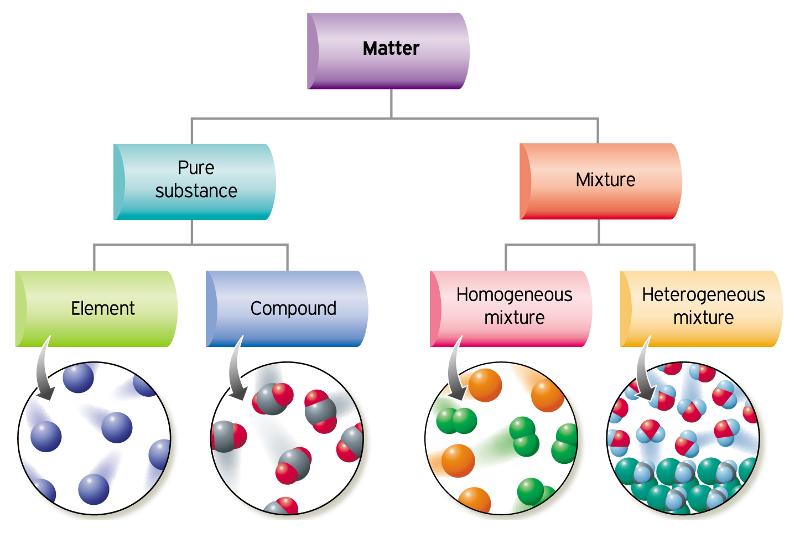
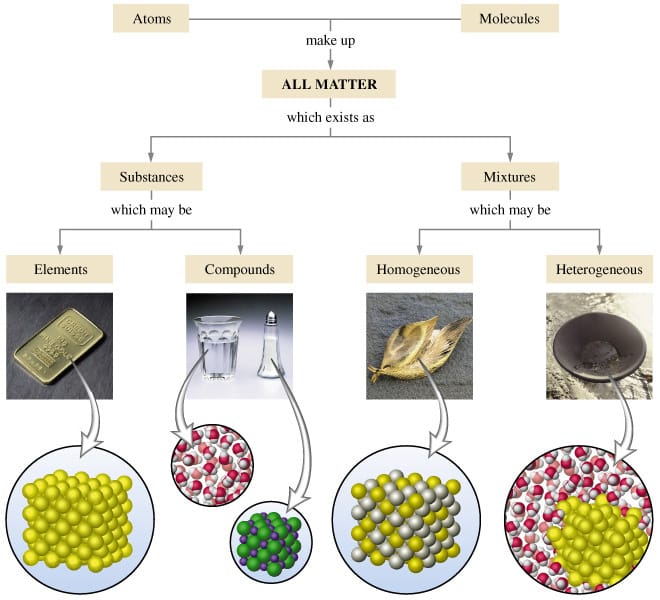
A homogeneous mixture has a uniform composition and appearance Individual substances that constitute a homogeneous mixture cannot be visually differentiated On the other hand, a heterogeneous mixture comprises two or more substances that can be distinctly observed, and even separated relatively easilyGive examples of an element, a compound, a heterogeneous mixture, and a homogeneous mixture Mixture A mixture is a material that has two or more different substancesUniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances or phases The three phases or states of matter are gas, liquid,



Sxxv4y Ycx9hem



Homogeneous Vs Heterogeneous Mixture
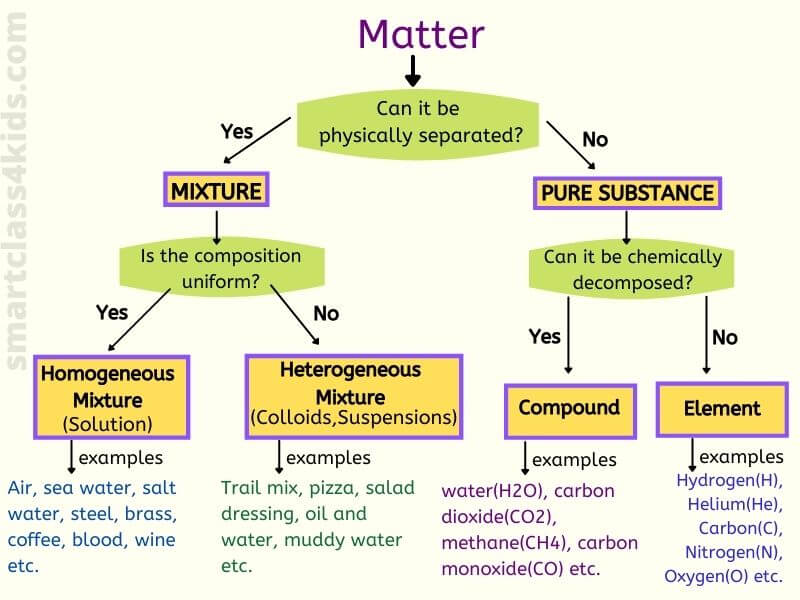
Practice Distinguishing between Heterogeneous & Homogeneous Mixtures with practice problems and explanations Get instant feedback, extra help and stepbystep explanationsHomogeneous mixtures 10 Heterogeneous and Homogeneous Mixtures A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughout There is only one phase of matter observed in a homogeneous mixture at a time Homogeneous Mixture vs Heterogeneous Mixture – Types of Solutions An unadulterated substance can be a component, or an intensify that are artificially homogenous in creation and can't be isolated by any physical methods A few instances of an unadulterated substance would be Iron Metal (Fe), Salt (NaCl) and so forth Most characteristic substances, and



Pure Substances And Mixtures Chemistry Quizizz



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube
A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture Vegetable soup is a heterogeneous mixture Vegetable soup is a heterogeneous mixture Any given spoonful of soup will contain varying amounts of the different vegetables and other components of the soupAnswer choices a mixture that is the same throughout a mixture that is made up of one type of atom a mixture that is made up of one type of compound a mixture that cannot be separated through physical changes The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties Homogeneous and heterogeneous are two different words




Classify Mixtures As Homogeneous Or Heterogeneous Worksheet




Homogeneous Or Heterogeneous Mixtures Practice Worksheet
The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughoutA homogeneous mixture is a mixture in which the composition is uniform throughout the mixture Heterogeneous Mixture A heterogeneous mixture is a mixture consists of two or more phases Elements Simplest form of matter can not be broken down Compounds Both of these types, homogeneous and heterogeneous, are the essentials of an environment You can simply analyze the nature of a mixture through its sample size If the sample shows multiple phases, it is definitely a heterogeneous mixture In an otherwise case, the mixture is entirely homogeneous



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Chemistry For Kids Chemical Mixtures
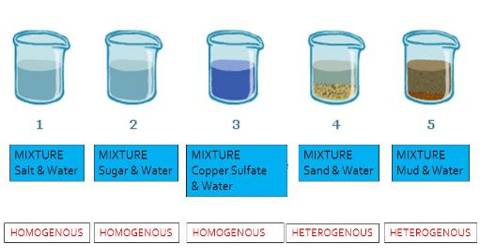
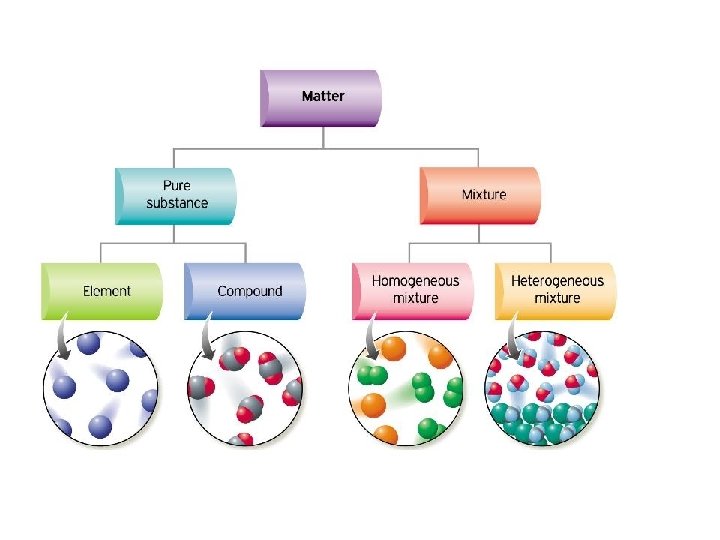
About heterogeneous mixture homogeneous mixture worksheet The heterogeneous mixture – homogeneous mixture worksheet with answer key is below The worksheet gives common examples of mixtures, in addition to some pure, unmixed substances Learn how to classify these examples of mixtures belowCharacteristics of mixtures Mixtures can be characterized by being separable by mechanical means eg heat, filtration, gravitational sorting, centrifugation etc Mixtures can be either homogeneous or heterogeneous' a mixture in which constituents are distributed uniformly is called homogeneous, such as salt in water, otherwise it is called heterogeneous, such as sandBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is




Solved Classify The Following Mixtures As Homogeneous Or Heterogeneous Begin Array Ll Text A Lemon Juice




Zline3bn7w 3ym
This means that the different components of the mixture cannot be distinguished from one another in some wayYou can see the DIFFERENT parts of the mixture from each other Homogeneous Mixtures Homogeneous mixtures mixtures that are evenly mixed! Some common examples of the mixture in our daily life are – air, milk, fruit juice, medicines, honey, tap water, brass, bronze, etc Air is a mixture of oxygen, nitrogen, carbon dioxide gases It also contains water vapor, dust particles, and traces of inert gases Mixtures are of two types – homogeneous and heterogeneous mixture




Homogeneous And Heterogeneous Mixtures Card Sorting Activity By Elly Thorsen




Question Video Characteristics Of Heterogeneous Mixtures Nagwa
Homogeneous mixtures and heterogeneous mixtures are different from one another The drinks that we buy in stores are homogeneous, whereas the mixtures which are not dissolved like sand and water are called heterogeneous mixtures It is interesting to note that homogeneous mixtures do not depict any tyndall effectTherefore, the individual components cannot be separately identified When allowed to stay undisturbed, the components of a homogeneous mixture do not settle down Solutions and colloids are the two main categories of aStart studying Homogeneous mixture vs Heterogeneous mixture Learn vocabulary, terms, and more with flashcards, games, and other study tools




Matter Mixtures Pure Substances Elements Compounds Heterogenous Ppt Video Online Download




Post Lab Exercises 1 Classify As Element Compound Chegg Com
Mixtures heterogeneous vs homogeneous A mixture is something that contains two or more substances Mixtures can be heterogeneous or homogeneous In a heterogeneous mixture, we can see the individual substances The individual substances can be separated easily A paella is a heterogeneous mixtureNo two muffins are identical, and thus the mixture is heterogeneous Homogeneous vs Heterogeneous Mixtures The differences between these two types of mixtures can be a bit difficult to distinguish Let's start by outlining some of the similarities Multiple substances, elements, or compounds – 2 or more;Heterogeneous vs Homogeneous Mixtures Heterogeneous vs Homogeneous Mixtures Presentation,Mixture Dualism in Blood activity, TeachEngineeringorg Note The slides are "animated," so clicking the mouse or keyboard advances the next text, image or slide



Homogeneous Mixture Experiment Qs Study




Homogeneous High Res Stock Images Shutterstock
A heterogeneous mixture has two or more phases and the components can be individually identified A homogeneous mixture is uniform; A mixture is a collection of different materials Take more than one thing, combine them and you have a mixture Mixtures can be further classified as either homogeneous or heterogeneous mixtures A homogeneous mixture is a mixture where the combined components is uniformly distributed throughout the mixture in a single phase Salt and water, for example, are homogeneous mixtures, as is sugar plus water As described by the dictionary of Chemistry, a heterogeneous mixture is a combination in which the constitution is not regular and smooth The elements are not homogeneous in their composition The components can't be dissolved readily



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science
Heterogeneous and Homogeneous Mixtures are discussed in this presentation High School chemistry, physical science, environmental science, earth systems, and Slideshare uses cookies to improve functionality and performance, and to provide you with relevant advertising4 $395 $295 PDF This 3 page worksheet covers the topics of elements, compounds and mixtures, homogeneous vs heterogeneous mixtures, extensive vs intensive properties, physical and chemical properties, physical and chemical changes, and states of matter Information is presented in a logical progression and has eQ Type of mixture that DOESN'T HAVE the same composition in every part



3




Identify The Following As An Element Compound Chegg Com
Heterogeneous vs Homogeneous Simone Young Ajoke Adigun Janice Lee Beverly Raynor Trevor Fockler 2 Definitions The prefix "homo" – indicate sameness Homogeneous – A homogeneous mixture has the same uniform appearance throughout Many homogeneous mixtures are commonly referred to as solutions 3In this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference betweenHomogeneous mixtures have uniform composition Heterogeneous mixtures have nonuniform composition




Properties Of Homogeneous Mixture The Difference Between Heterogeneous And Homogeneous Mixtures



Classifying Matter Schoolworkhelper
Q What is a homogeneous mixture?Start studying Homogeneous vs Heterogeneous Learn vocabulary, terms, and more with flashcards, games, and other study toolsEverything looks the same You don't see different parts Solutions



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture Bitwise Academy
Homogeneous mixtures have only one phase of matter, be it solid, liquid or gas Heterogeneous mixtures have more than one phases of matter It should be reminded here, that non mixing liquids are not considered 'one phase' For solutions in a trButtermilk is another example of a heterogeneous solution On the other hand, an example of a homogeneous mixture is a salt water solution or a solution of water, with sugar dissolved in it The appearance of the water, despite dissolution of salt/sugar, is uniform Other examples are white vinegar and corn oil Homogeneous vs Heterogeneous Mixtures Mixtures can be either heterogeneous or homogeneous Unlike a heterogeneous mixture, a homogeneous mixture is a mixture that is uniform throughout;




Heterogeneous And Homogeneous Mixtures With Examples Study Guide Brighthub Education




Ppt Classify The Following Mixtures As Either Homogeneous Or Heterogeneous Powerpoint Presentation Id
Homogeneous Vs Heterogeneous Mixture In chemistry, we can have two types of mixtures homogeneous mixtures and heterogeneous mixtures Homogeneous mixture Blended so thoroughly, it looks like one substance – Uniform composition Heterogeneous mixture Not thoroughly blended, so you can see and pick out an individual part of the mixture The homogeneous mixture is only in the one phase of matter, whereas heterogeneous mixture is always in two or more than two different phases of matter Solutions are termed as the homogeneous mixtures, on the other hand, suspensions or colloids are termed as the heterogeneous mixturesMixtures formed by combining two or more materials either form homogeneous or heterogeneous mixturesHomogeneous mixture comes from homo (th




Homogeneous And Heterogeneous Mixtures Learn How To Solve Practice Exam 1 Q 1 Youtube



1




Homogeneous Or Heterogeneous Worksheet




Difference Between Homogeneous Mixture And Heterogeneous Mixture



Mixture




What Is A Homogeneous Mixture Definition And Examples




Classification Of Matter Chemistrygod



Matter Mixtures Mixtures Many Substances In Nature Are




Homogeneous Mixture And Heterogeneous Mixture Is Matter Around Us Pure Chemistry Class 9 Youtube




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards
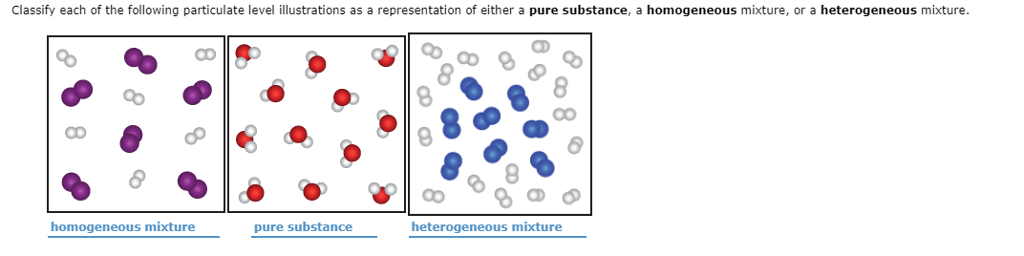



Classify Each Of The Following Particulate Level Chegg Com
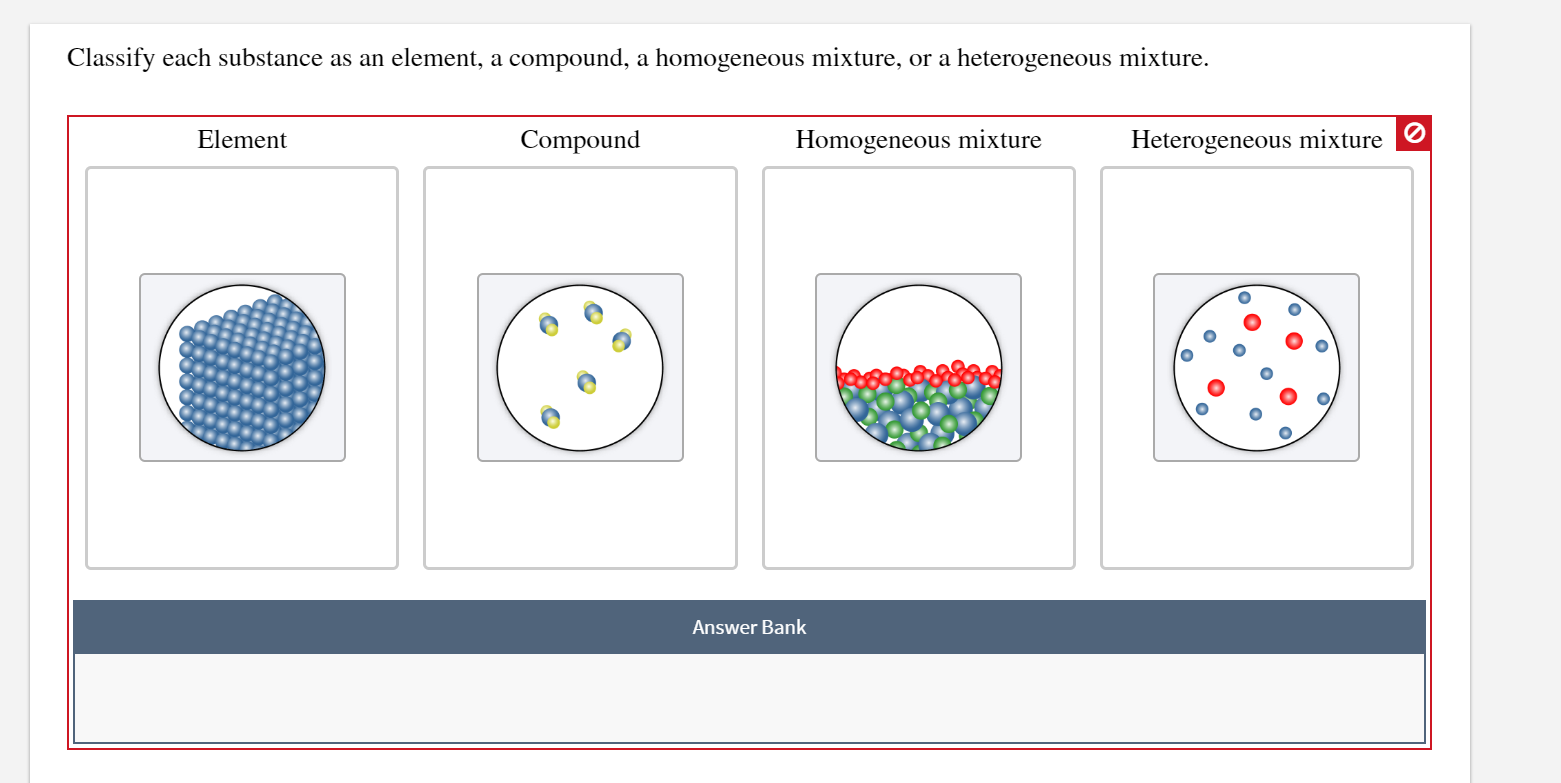



Classify Each Substance As An Element A Compound A Chegg Com



Heterogeneous And Homogeneous Mixture Differences Videos Examples




Mixturesandsolutions



What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples
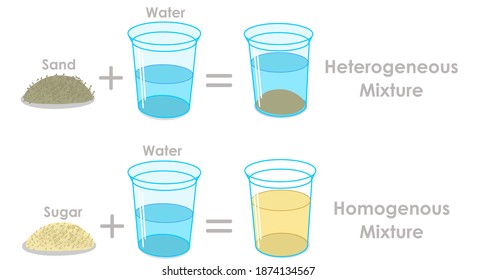



Mixtures Hd Stock Images Shutterstock




Classify Each Of The Following Particulate Level Chegg Com
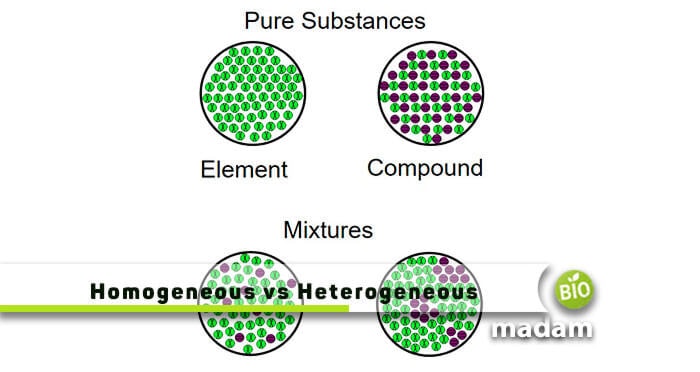



Difference Between Homogeneous Heterogeneous Mixtures Biomadam



Heterogeneous Vs Homogeneous Mixtures Sovannary
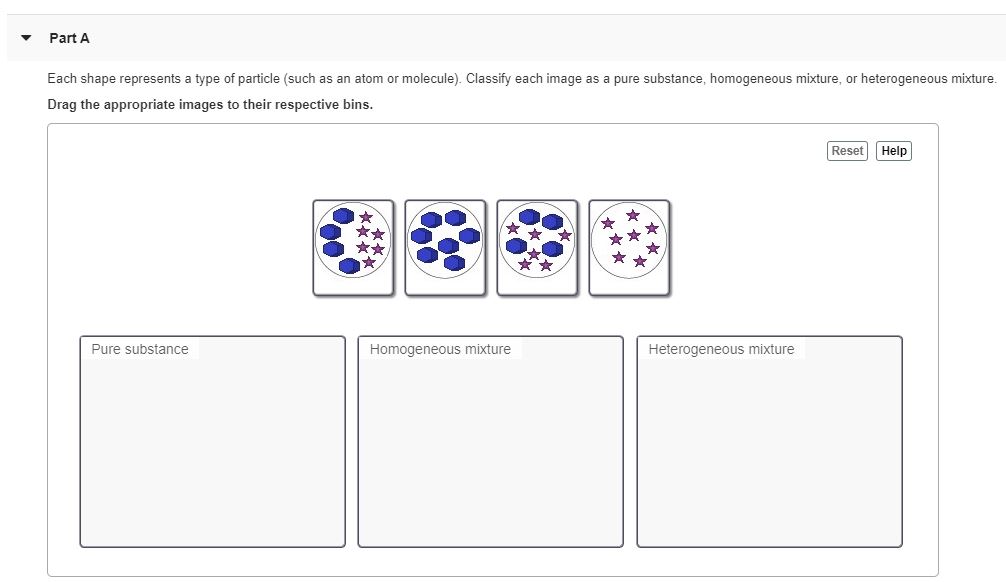



Answered Each Shape Represents A Type Of Bartleby




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
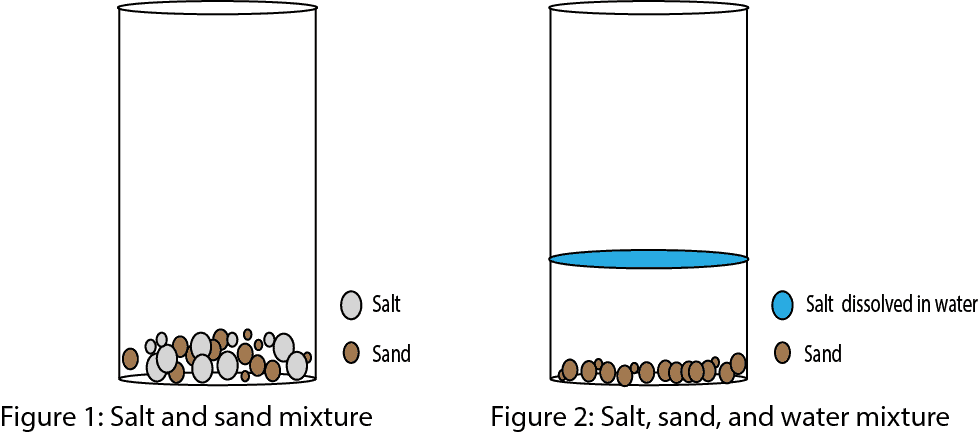



What S A Mixture How Does Heterogeneous Differ From Homogeneous Mixture And How Can Mixtures Be Separated



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Chapter 1 Br Section A Br Some Basic Definitions




Ch 12 1 Types Of Mixtures Heterogeneous Vs Homogeneous Mixtures Heterogeneous Mixture Mixture Does Not Have A Uniform Composition Ex Milk And Soil Ppt Download




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



What Is A Gaseous Example Of A Heterogeneous Mixture Quora



Mixture
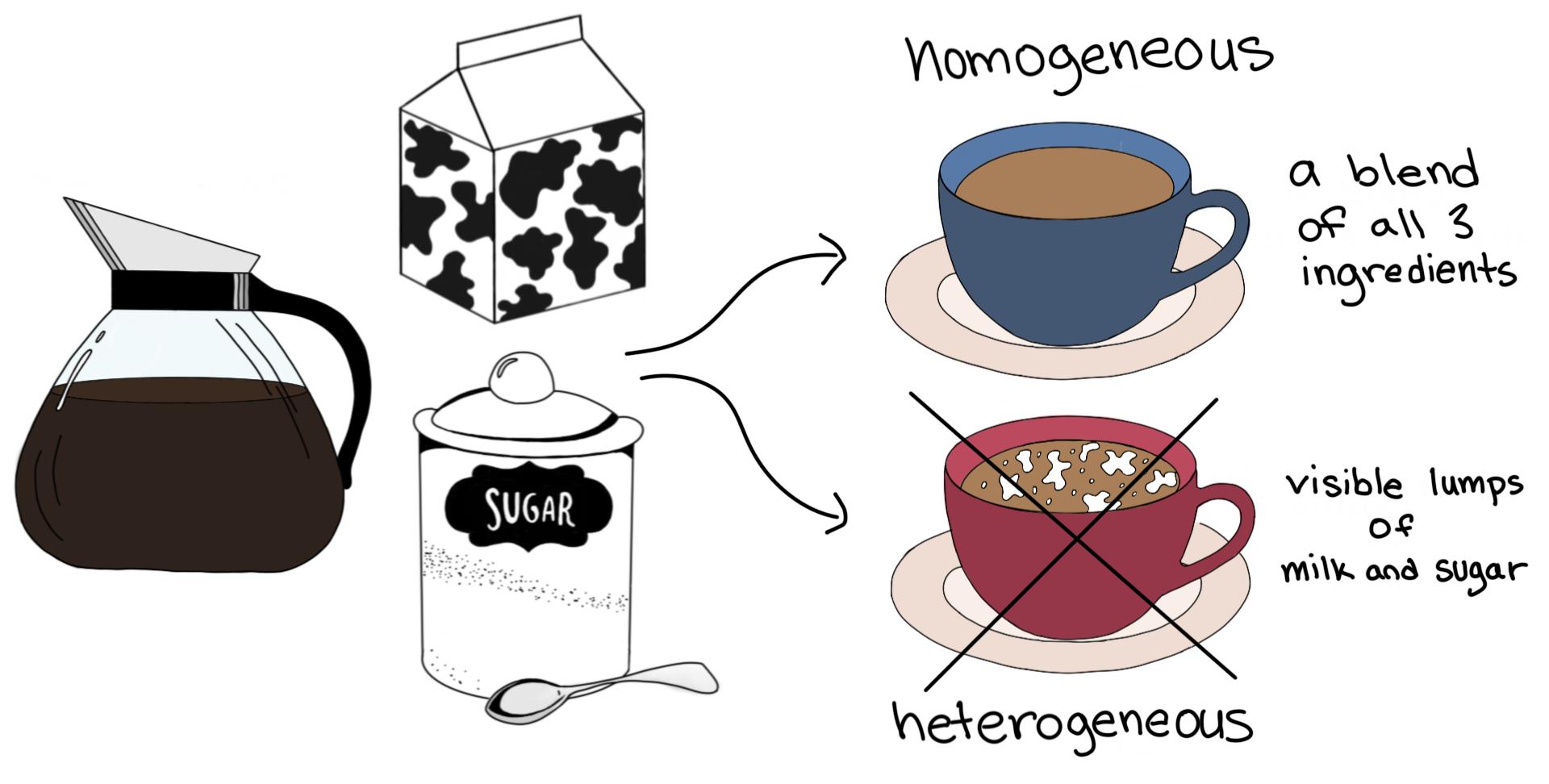



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
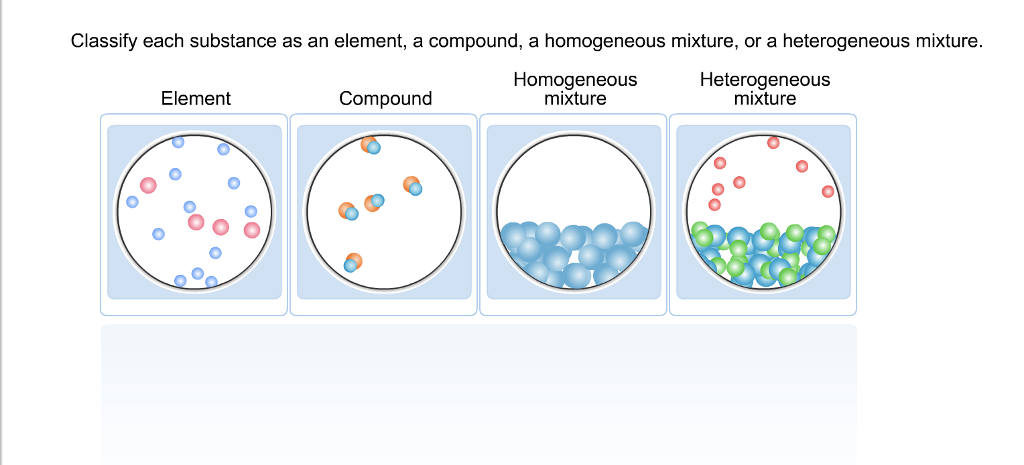



Classify Each Substance As An Element A Compound A Chegg Com
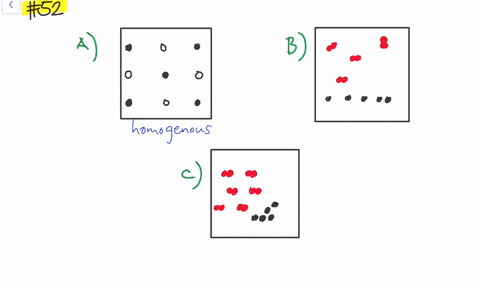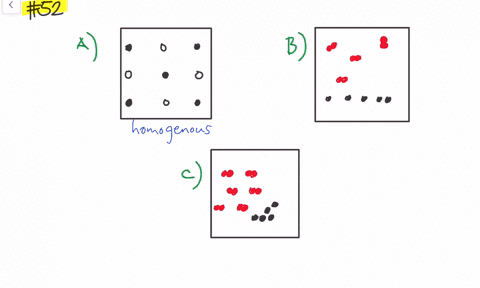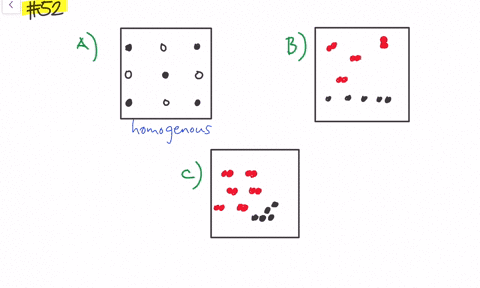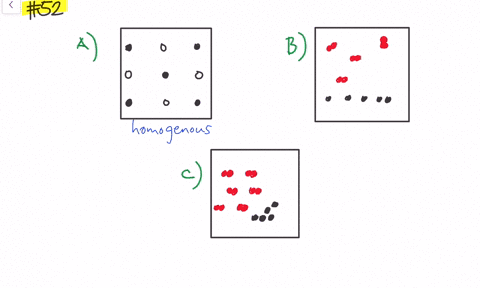



Solved Give Three Examples Each Of Heterogeneous Mixtures And Homogeneous Mixtures
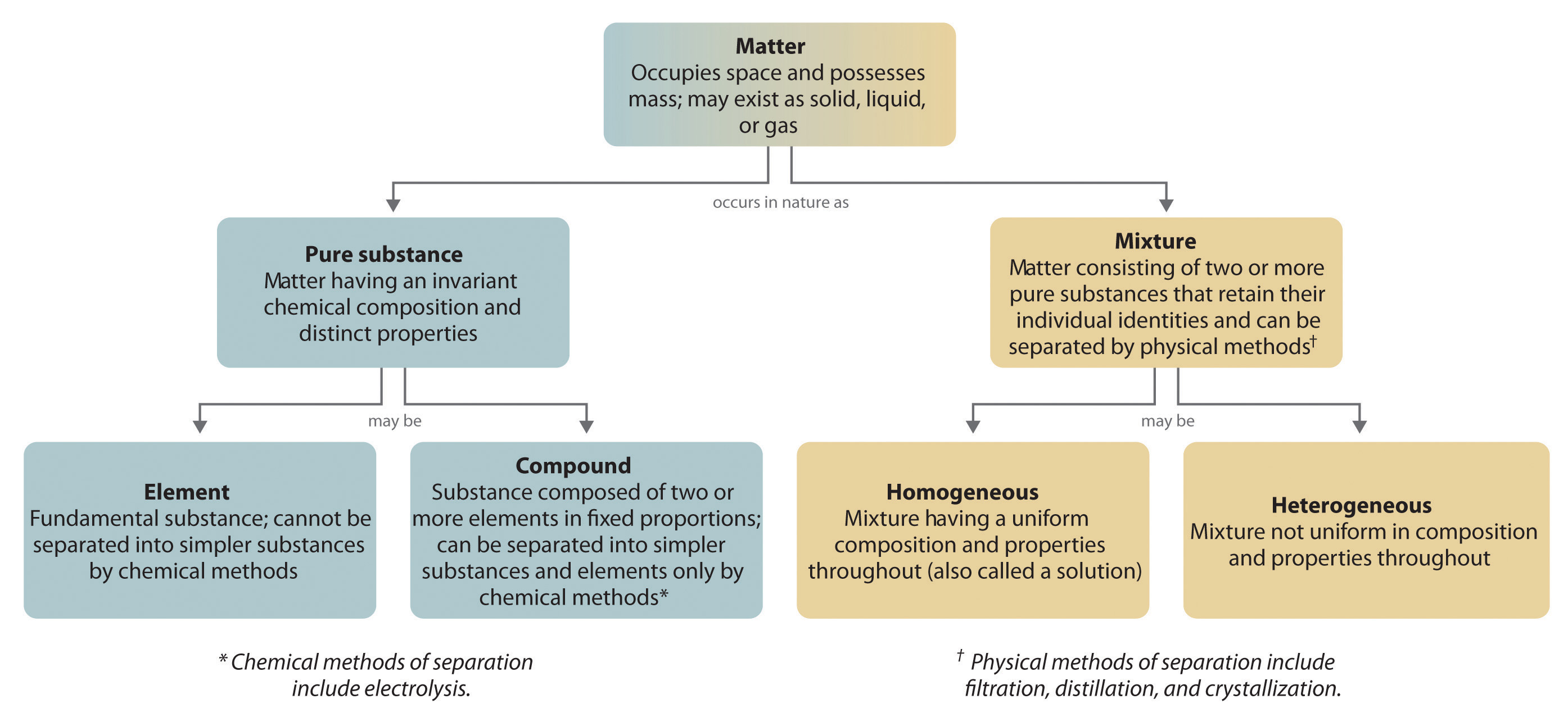



A Description Of Matter
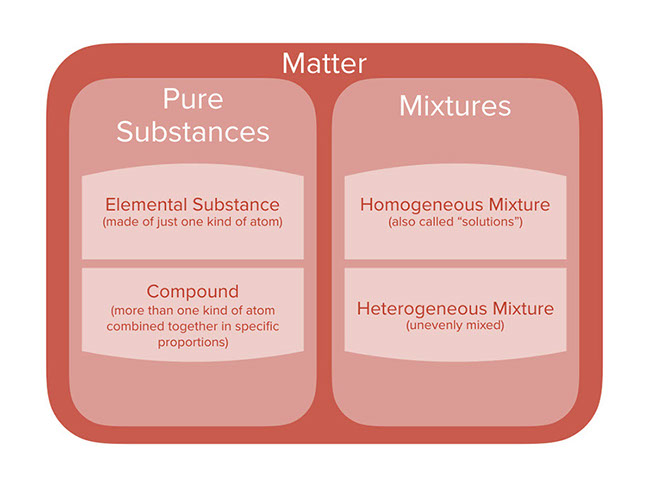



Lesson Categories Of Chemicals And Mixtures



Solved Give Three Examples Each Of Heterogeneous Mixtures And Homogeneous Mixtures



3




Heterogeneous Mixture And Homogeneous Mixture Youtube




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry




Classify The Following Mixtures As Homogen Clutch Prep
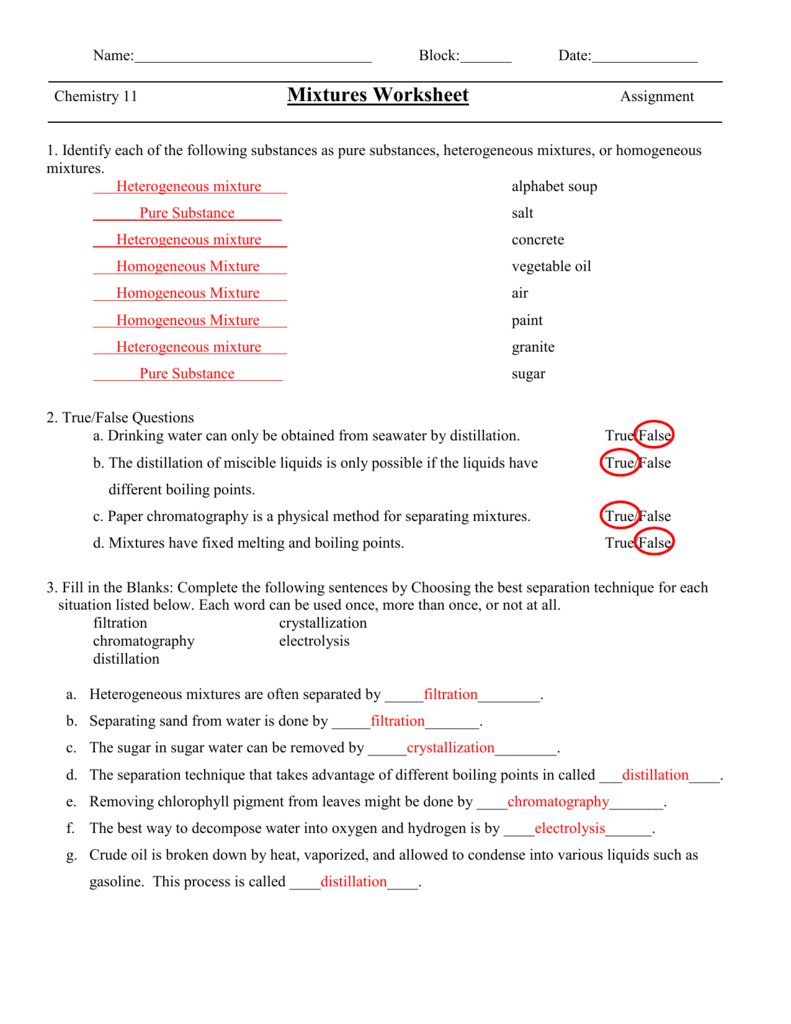



Mixtures Worksheet




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube



Is Air Considered To Be A Homogeneous Or A Heterogeneous Mixtures Quora
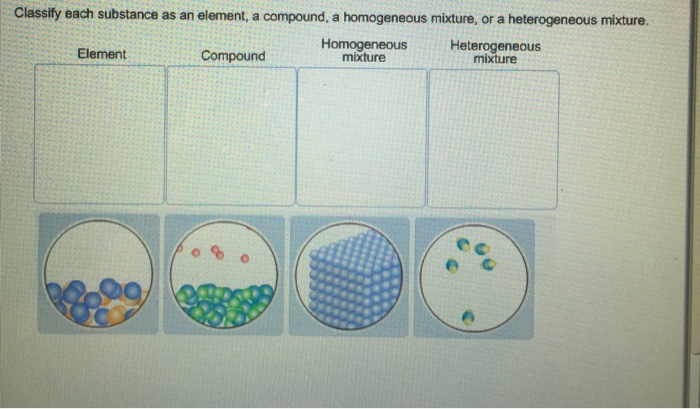



Classify Each Substance As An Element A Compound A Chegg Com



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Heterogeneous Vs Homogeneous Mixtures How Are They Different Homogeneous Mixtures Have Uniform Composition Heterogeneous Mixtures Have Non Uniform Ppt Download



Explain I Pure Substances Vs Mixtures Uths Demo Course
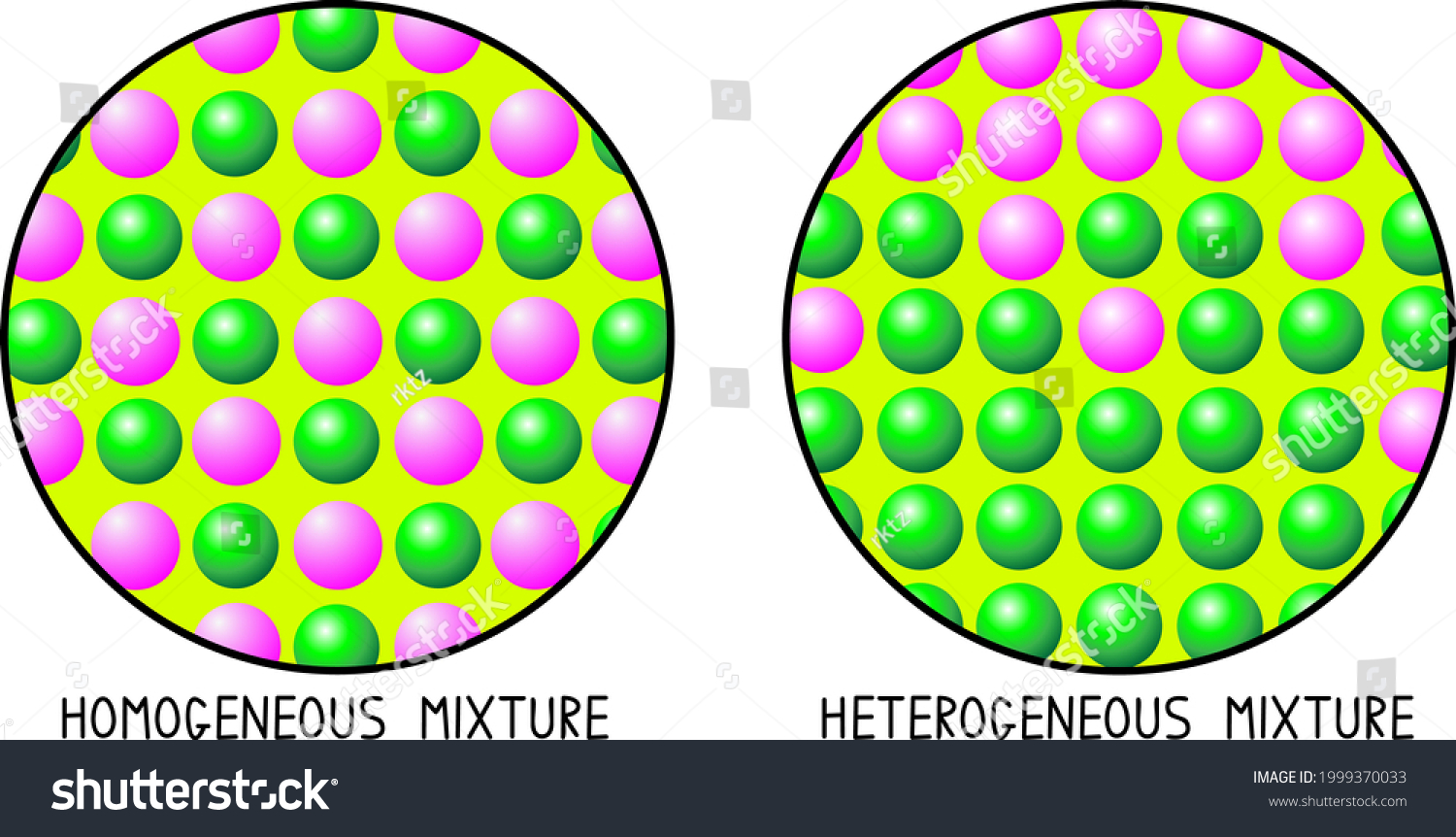



Homogeneous Mixture Vs Heterogeneous Mixture Particle Stock Vector Royalty Free




What Is A Heterogeneous Mixture Definition And Examples




Compound Vs Mixture Difference And Comparison Diffen




1 Classify The Following Liquid Mixtures As Chegg Com
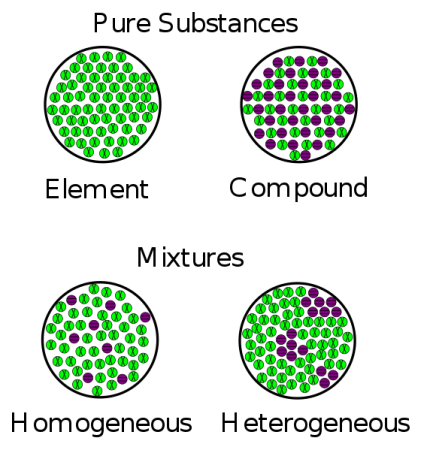



Heterogeneous Mixture Definition Science Trends



Heterogeneous Vs Homogeneous Solutions Chapter 12 Solutions




Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture Venn Diagram Examples Venn Diagram Template




Warning This Slide Show Will Make You Hungry




Heterogeneous Mixtures Homogeneous Mixtures By Ms Cunningham



Www Austincc Edu Mohan Documents 04 Worked Examples Pdf
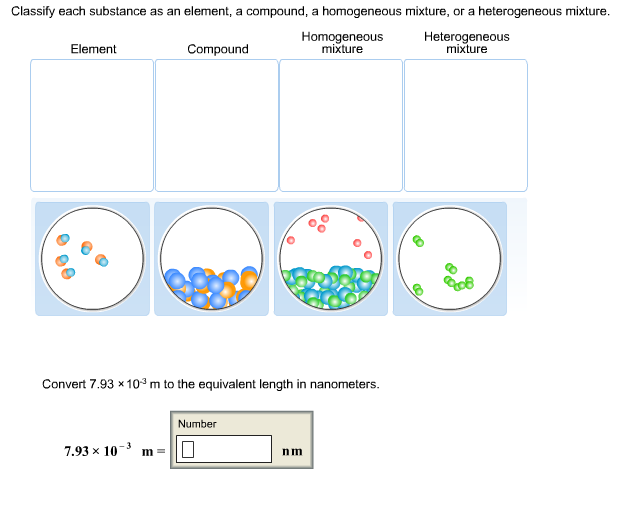



Classify Each Substance As An Element A Compound A Chegg Com




Elements Compounds And Mixtures Oh My Ppt Download




Classify The Following Mixtures As Homogeneous Or Heterogene Clutch Prep




If It Is A Mixture Classify It As Homogen Clutch Prep




10 Examples Of Mixtures
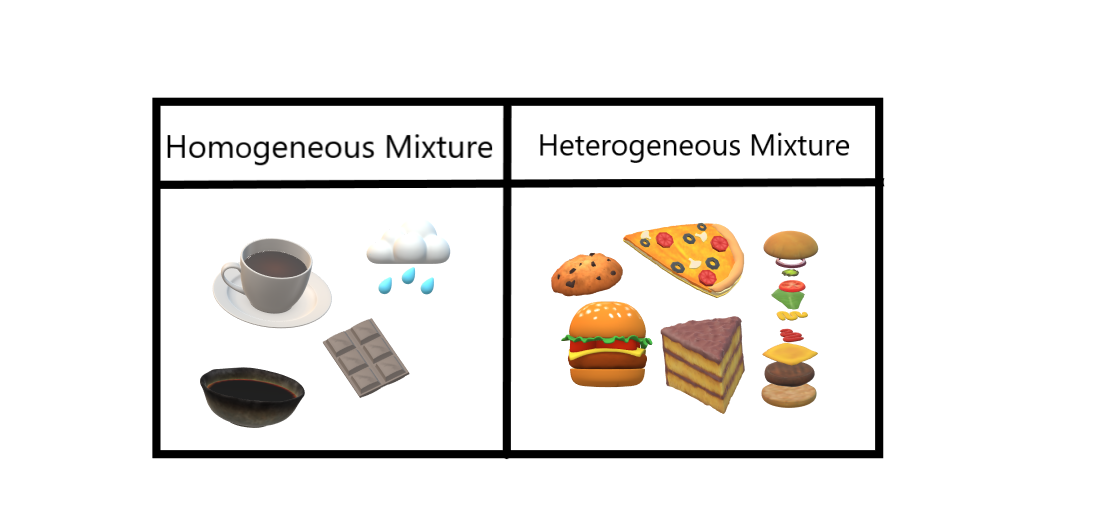



Homogeneous And Heterogeneous Mixtures Geeksforgeeks
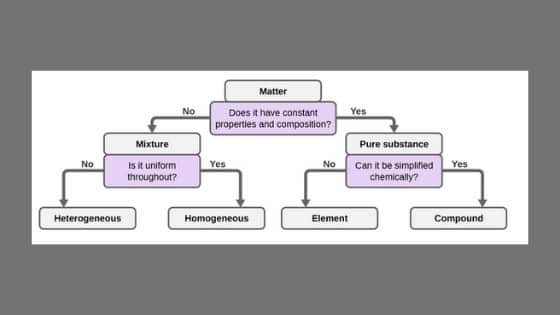



Homogeneous Mixture And Heterogeneous Mixture Ncert Books




Homogenous Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube




Answer In Homogeneous Or Heterogeneous What Type Of Mixtures Are Separated By The Brainly In




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Solutions And Mixtures Flashcards Quizlet




Homogeneous Mixtures Vs Heterogeneous Mixtures By Brianna Dearmon



1
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Shutterstock Puzzlepix
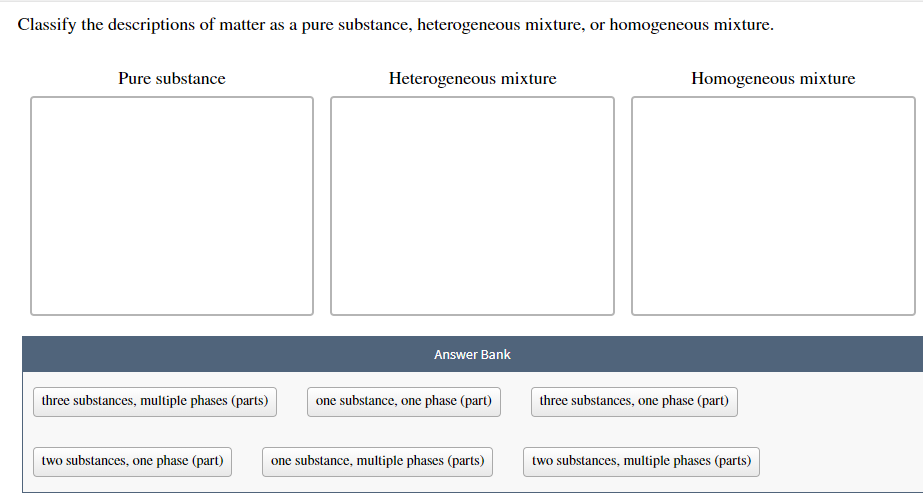



Classify The Descriptions Of Matter As A Pure Chegg Com
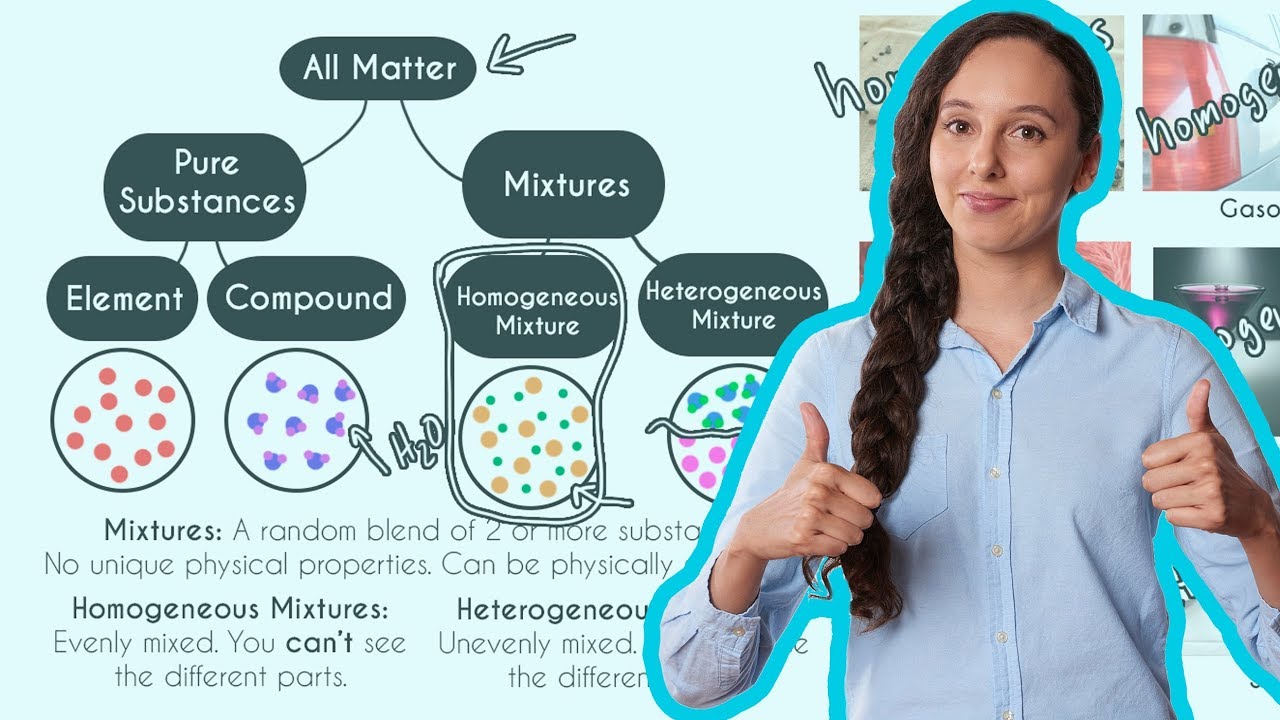



Homogeneous And Heterogeneous Mixtures Youtube




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous




Types Of Mixtures Video Khan Academy




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures



0 件のコメント:
コメントを投稿